
Would you want a robot to take your blood sample? The inventors of Aletta, the world’s first autonomous robotic phlebotomy device, think you might – especially when you hear how accurate it is. And, with phlebotomists in increasingly short supply, visiting the robot assistant might be preferable to having a long wait time for a short procedure.
AI-driven automation is now commonplace in pathology labs – but could it also represent the future of clinical care? We connected with Toon Overbeeke, CEO and Co-Founder, and Luuk Giesen, Chief Medical Officer, of Vitestro to find out more about the technology and how patients react to it.
What inspired the development of Aletta?
Toon Overbeeke: The idea for Aletta was inspired by a good friend of mine. His father was being treated for cancer and it was proving very difficult to draw blood from his veins. After a series of missed punctures my friend asked me, “Is it not possible to improve the blood drawing procedure with technology?”
I founded the company in 2017, with the aim of achieving a better experience for patients with veins that are difficult to puncture. When I talked with labs and hospitals to try to understand the clinical needs, many of them were super excited about the idea.
The main reason for the excitement was linked to the problem of staff shortages – skilled phlebotomists are becoming difficult to find. But the other reason was that blood drawing is quite an error-prone procedure. Hemolysis rates depend highly on the skill and experience of the phlebotomist – they tend to be higher with less experienced practitioners or in emergency medicine, where staff don’t have as much practice.
The labs we spoke to were very excited about the possibility of standardizing the phlebotomy process to reduce errors and mitigate staff shortages.
What challenges did you encounter in creating an autonomous robotic phlebotomy device?
Luuk Giesen: The most difficult thing was to automate firstly vein detection and secondly needle insertion. That was the initial focus and, in 2019, I was the first patient to have my blood drawn by the first prototype.
That was very exciting, but we knew that the labs wanted automation of the complete process. We had to work out how to automate everything from application of a tourniquet, to securing a bandage and handling the collection tube – and then test, develop, and iterate each aspect.
The next challenge was ensuring a good user experience. We wanted the user to understand where and how to place their arm, and to ensure their comfort throughout the procedure.
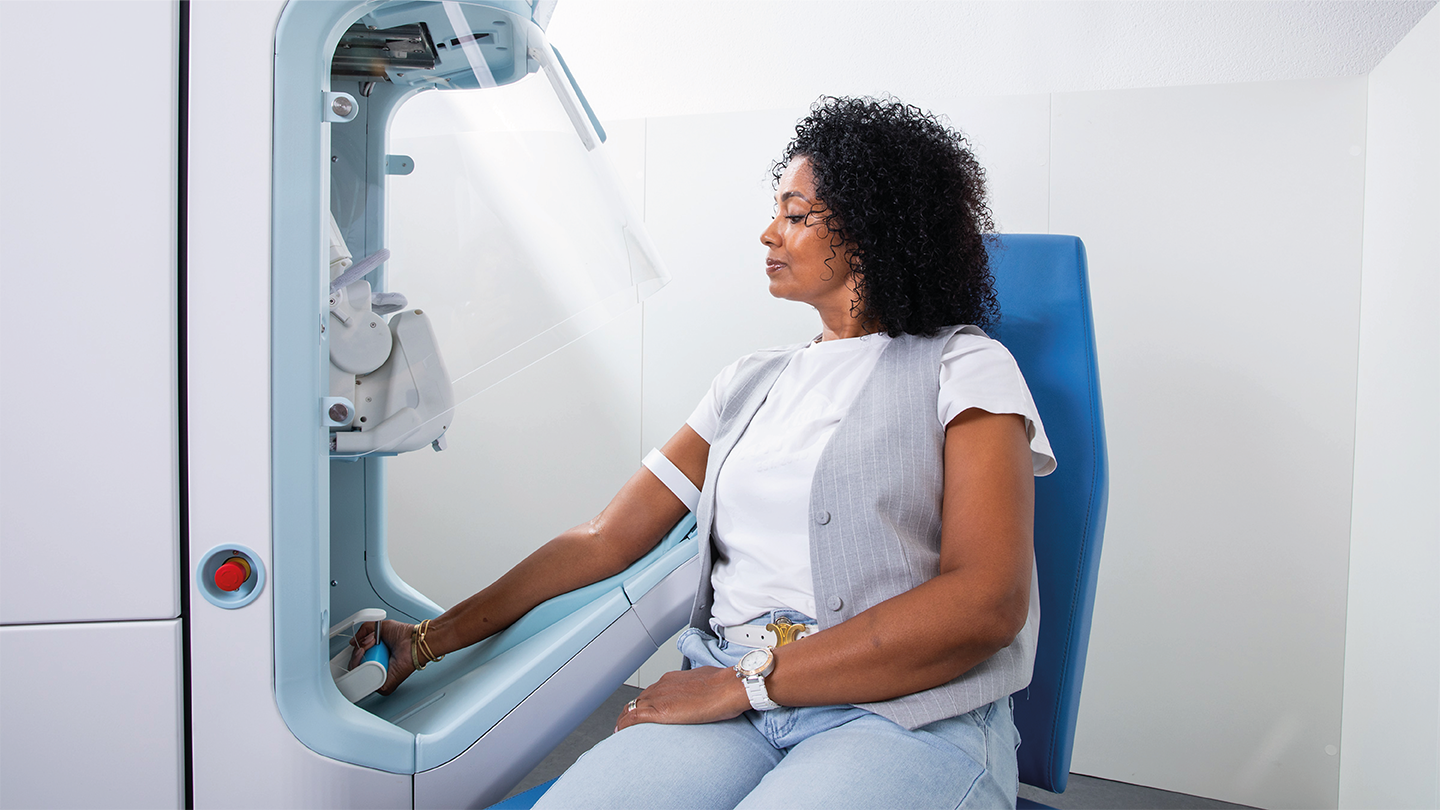
What are the technologies driving the system?
TO: We use two different technologies to find the vein. The first is infrared light, which is absorbed by hemoglobin in the blood so that the vein appears black. That gives an approximate location for the vein, but lacks information about its depth, size, and quality.
An ultrasound probe then moves over the arm and produces an image of the vein. Doppler enhancement distinguishes arteries from veins, so the system will only ever puncture a vein.
AI algorithms assess the ultrasound image and determine the precise location and depth of the vein, and its suitability for venipuncture. Those data then guide the robotic arm to position the needle very precisely in the arm of the patient.
What steps were taken to ensure Aletta enhances patient comfort and safety?
LG: The system was designed for a broad patient population, including those with difficult venous access. In those patients it can be difficult for a phlebotomist to both see and feel the vein – the most suitable vein might be located deeper than they can detect. Our ultrasound-guided system can locate those deeper veins quite easily, which could improve the patient experience for some patients.
Another thing that’s important for the patient experience is that the needle is only inserted if the device is certain that the vein is suitable for puncture. The algorithm rejects any that are too small or deep – only when all the parameters are met is the needle inserted.
In terms of comfort, we wanted all the components to move in a smooth and controlled manner, to maximize trust in the system. Patients also told us they don’t like to feel the needle moving in the skin when the collection tube is switched or removed. Our system uses a butterfly needle that is kept still through the whole procedure. In general, patients indicate that the automated venipuncture feels less painful than the manual procedure.
We’ll be able to elaborate on the user experience later this year, after the first results of our clinical trials are published. But those results do look very positive.
How does Aletta address common preanalytical errors in blood collection?
LG: In phlebotomy, hemolysis can be caused by everything from the way the needle is inserted into the vein to the manner of the inversion of the collection tube. Therefore, it follows that the greater the number of steps that can be standardized, the better the quality of the blood sample will be.
TO: Our studies show that hemolysis rates in blood collected with the Aletta system are lower than in manual blood draws. The exact numbers will be published in our forthcoming paper, but it’s a huge difference.
What considerations were made to ensure Aletta integrates seamlessly into clinical workflows?
TO: We developed Aletta with the help of a design agency that specializes in human device behavior. From a patient adoption perspective, the system needs to be open so you can see where to position your arm and how the vein is detected. However, patients don’t want to see the needle going into your skin – if you look at a phlebotomy department, the majority of patients look away during the insertion. So we had to balance an open design – which is more complex from a technology perspective – with one that shields the patient from the actual needle puncture.
Then we incorporated curved designs into the unit to make it as attractive as possible. We also tried to highlight the most important parts of the device so it’s clear to the patient where and how to place their arm. Whilst there will always be a supervisor available, we want experienced patients to be able to use the device unaided.
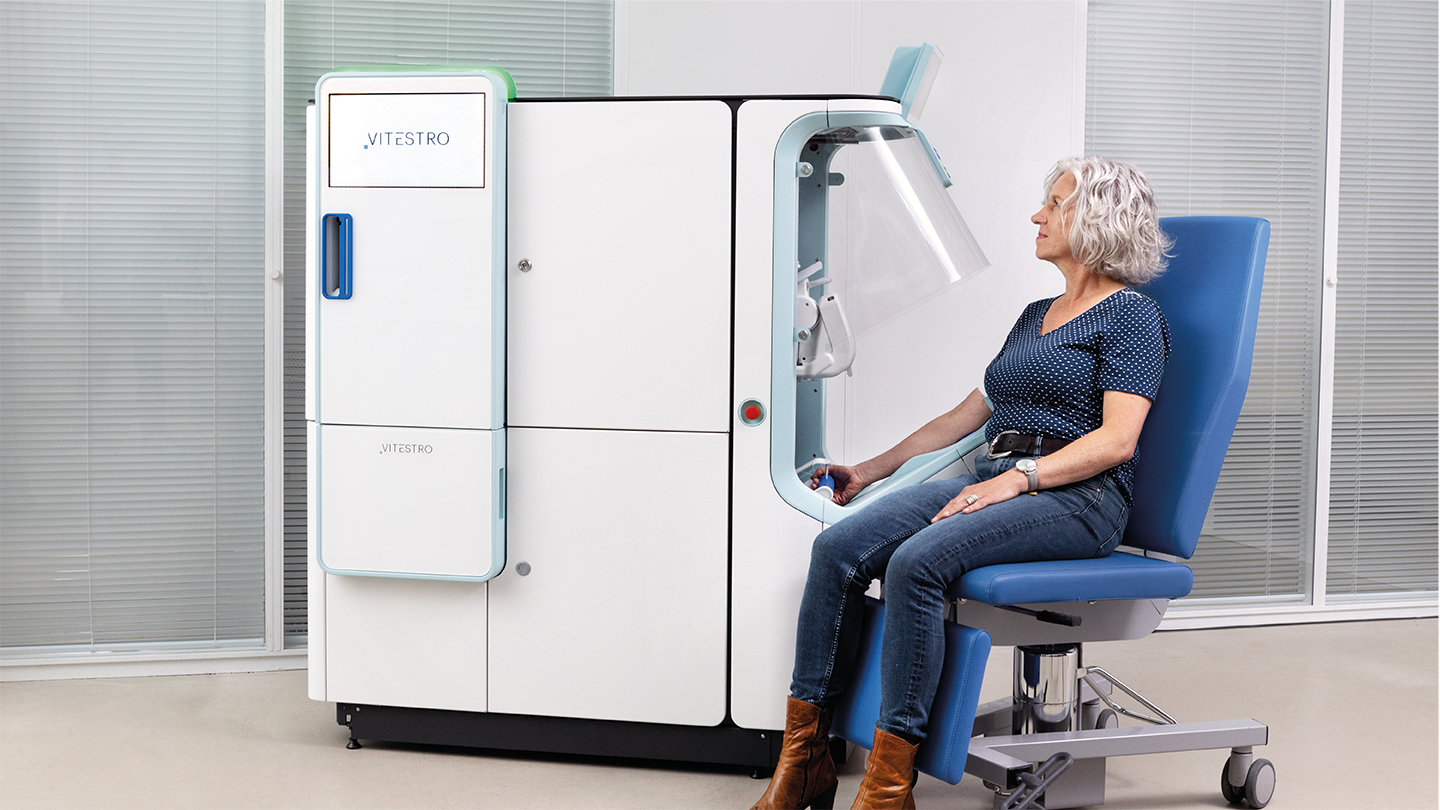
What feedback have you received from healthcare professionals and patients?
LG: The professionals we work with are pathologists and lab specialists. They are used to automation in the lab, so they appreciate the technology in our system. Whilst this is a medical device, not an analyzer in the lab, they seem quite impressed that this system can reliably and consistently collect a blood sample, with low hemolysis rates.
The patients that we speak to are generally very positive. Nearly all say that the automated system is an acceptable method of blood collection when compared to the manual procedure.
What regulatory challenges did you face in bringing Aletta to market?
TO: Aletta received a CE Mark last year, so we now have a commercial presence in Europe. That was challenging because we had to meet the requirements of the Medical Device Regulation (MDR), which replaced the Medical Device Directive a couple of years ago. The MDR is more stringent and requires more clinical evaluation.
Although we were one of the first companies to have a product CE marked under the MDR, the process was relatively smooth. We planned everything very well and achieved clearance even quicker than we expected.
How do you foresee its adoption across different healthcare settings?
LG: Our initial focus is on high-volume phlebotomy sites, with at least 100 patients per day. That’s also because we want to give patients the choice between manual and automated phlebotomy at this stage.
In the future, when the system is further improved and refined, we expect to be able to place these systems in lower volume blood draw sites.
Beyond phlebotomy, do you see potential for AI-driven robotic automation in other areas of laboratory medicine?
OT: Right now, we are now fully focused on bringing Aletta to the market and making it a huge success. But I think there are definitely many more applications for this technology. Blood donation, for example, would definitely benefit from a standardized procedure.
We haven’t decided yet what to pursue, but there are huge opportunities for us in the future.




