
New research, presented at the AD/PD 2025 meeting in Vienna, has revealed a potential biomarker for diagnosis and monitoring of Parkinson’s disease (PD). The study – a collaboration between Merck and the Michael J. Fox Foundation for Parkinson’s Research (MJFF) – shows an initial proof of concept for a novel α-synuclein positron emission tomography (PET) tracer that visualizes α-synuclein pathology in the brain of patients with Parkinson’s disease.
We connected with Robert E. Drolet, Senior Principal Scientist, Neuroscience Department, Merck, to learn more about the implications of the results for Parkinson’s diagnostics.
What are the unmet needs in Parkinson’s diagnostics that this research is attempting to address?
There is a critical need for disease progression biomarkers in PD to support clinical trial efficiency in drug development. At present, there is no single test that a doctor can use to make a definitive diagnosis of PD. Instead, they rely on present and past symptoms, medical history and in-office exams to inform a diagnosis.
This proof-of-concept study is an important step to developing a much-needed biomarker for use in the diagnosis and evaluation of the progression of PD. Importantly, it also provides a potentially objective means to evaluate the efficacy of investigational medicines for the treatment of PD.
Biomarkers can also help improve how we design clinical trials because we’ll have improved ability to ensure participants meet the trial parameters.
What were the key findings of the study?
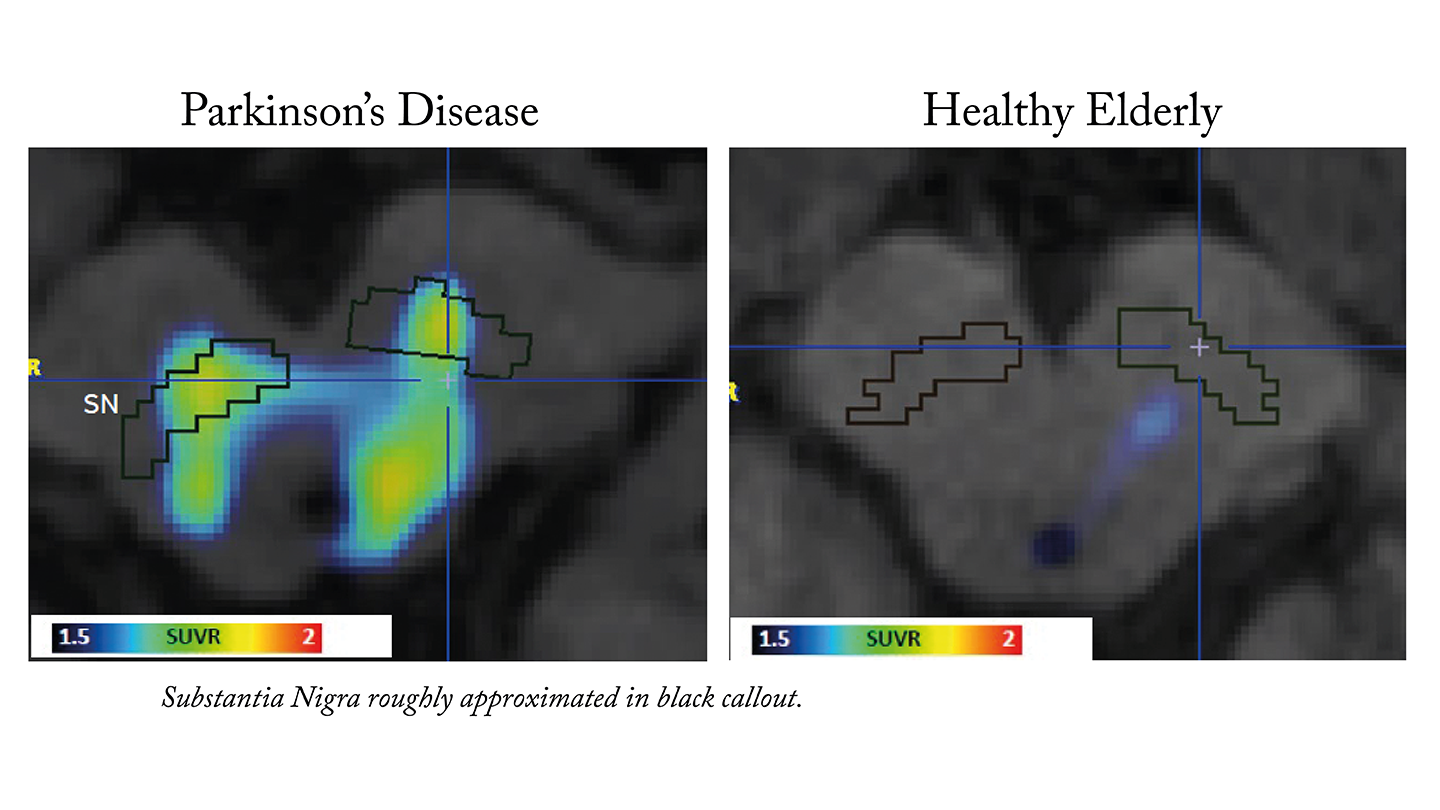
Funded in part by an award from the MJFF for Parkinson’s Research Ken Griffin Alpha Synuclein Imaging Competition, Merck presented a study at AD/PD 2025 discussing how a novel α-synuclein PET tracer, [11C]MK-7337, demonstrates proof-of-concept for imaging α-synuclein pathology in Parkinson’s disease patients.
[11C]MK-7337 produced the first-in-human evidence of imaging α-synuclein pathology in three regions of interest in Parkinson’s disease patients:
Hyposmic patients showed elevated signals in the olfactory epithelium.
Hyposmic PD patients showed elevated signals in the substantia nigra and brainstem subregion, and no evidence of synuclein pathology in the amygdala.
This shows that the tracer binds preferentially to pathological α-synuclein versus healthy tissue.
While the short 20-minute half-life of 11C limits broad application of [11C]MK-7337, the headline from this study is that its success heralds the potential for identifying a biomarker for Parkinson’s that will improve our understanding the disease biology, the selection of therapeutic targets, and our ability to execute clinical trials.
What are the implications of these findings for patients with Parkinson’s disease?
Ten years ago, researchers were struggling to design clinical trials and develop therapies for Alzheimer's disease – until progress was made to identify how Tau and amyloid β PET ligands could be integrated into research. This discovery really propelled Alzheimer’s research forward across the scientific community.
Similarly, we are hopeful that an α-Synuclein PET tracer is the steppingstone that excites the research community and advances research approaches for Parkinson’s disease.
What are the implications for clinical trial design?
Clinical trials for neurodegenerative diseases often face high failure rates due to the complexity of disease biology, limited validated targets, the challenge of identifying reliable biomarkers to evaluate benefit and risk, and subjectivity of endpoints. This makes monitoring the adequacy of participant interviews and ratings an essential part of strong research methodology.
This is especially true in Parkinson’s disease, which lacks an objective measurement tool to determine a participant’s fit for the clinical trial design. If we can more fully develop an α-synuclein PET tracer biomarker for Parkinson’s disease, we can be more targeted in our selection of study targets.
What’s next for this research?
This is an exciting possibility for Parkinson’s disease researchers. We look forward to continuing our collaboration with the MJFF for Parkinson’s research and other investigators to determine the potential of an α-synuclein PET tracer biomarker.
Merck is sharing the full study design and results to encourage scientific dialogue on how to advance this line of study to benefit Parkinson’s disease research in the future. Looking ahead, Merck will advance a next candidate [18F] PET tracer into clinical study in 2025 and anticipates demonstration of proof-of-concept in 2026. We are also open to collaborating with other investigators to further explore MK-7337, provided they have [11C] capabilities.




